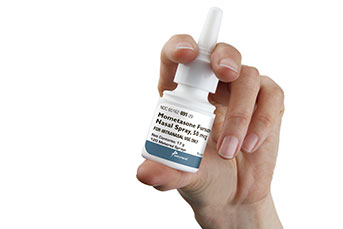
Bridgewater, NJ (USA), April 11, 2017 – Amneal Pharmaceuticals LLC has launched mometasone furoate nasal spray, the company’s first pharmaceutical product in spray form. Mometasone furoate, available in 50 mcg/spray strength, is an AB-rated therapeutic equivalent to Nasonex®.
Amneal’s generic comes packaged in a white, high-density, polyethylene bottle fitted with a white, metered-dose, manual spray pump and turquoise cap. Each 17-gram bottle delivers 120 sprays.
Manufactured in Branchburg, NJ, the product began shipping April 4th through wholesalers, distributors and directly to the trade.
“This launch marks a new milestone for us,” explains Jim Luce, EVP Sales & Marketing. “As the first nasal spray product from Amneal, it stands as yet another example of how we continue to expand into new and more complex dosage forms. Metered dose inhalers, transdermal patches and chewable tablets are just of few of those in the pipeline that we are excited to bring to market, significantly expanding our product offering.”
Click here to view full prescribing information for mometasone furoate nasal spray.
Annual U.S. sales of brand and generic Nasonex® were $653 million according to December 2016 IMS market data.