Specialty R&D


Our Specialty portfolio delivers innovative medicines focused on meeting important medical needs across therapeutic categories, including neurology (Parkinson’s Disease, Migraine), endocrinology (Hypothyroidism), and other therapeutic areas.
We are focused on the continued growth and expansion of our product portfolio through internal development as well as through acquisitions and late-stage and next-generation product partnership opportunities.
Clinical Activities(1)
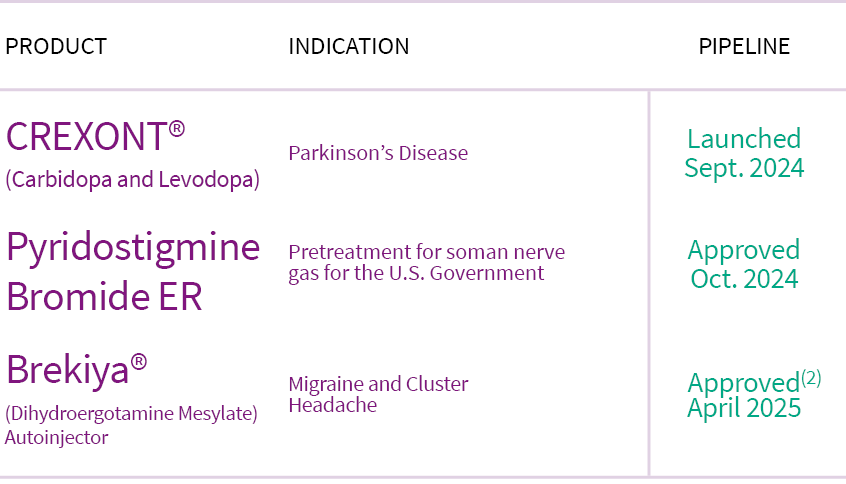
Product
Indication
Estimated Launch
Crexont® (Carbidopa and Levodopa)
Parkinson’s Disease
Launched Sept. 2024
Pyridostigmine Bromide ER
Pretreatment for soman nerve gas for the U.S. Government
Approved Oct. 2024
Brekiya® (Dihydroergotamine Mesylate) Autoinjector
Migraine and Cluster Headache
Approved April 2025 (2)
Note: Data as of August 5, 2025
(1) Pipeline may include investigational products not approved by FDA. Any such expected launch is subject to certain assumptions and factors, many of which are outside our control, such as regulatory approval, and may be subject to change.
(2) Pending Launch October 2025
