Research & Development


Our R&D strategy is led by a world-class in-house development team and supplemented by creative product development partnerships with external stakeholders. The focus of our R&D strategies is ensuring the ongoing innovation of important and value-generating products across our portfolios.
A proven track record of innovation for patients
The graphic below reflects the timing of when a new product in each dosage form category was initially launched by Amneal or is expected to launch(1).
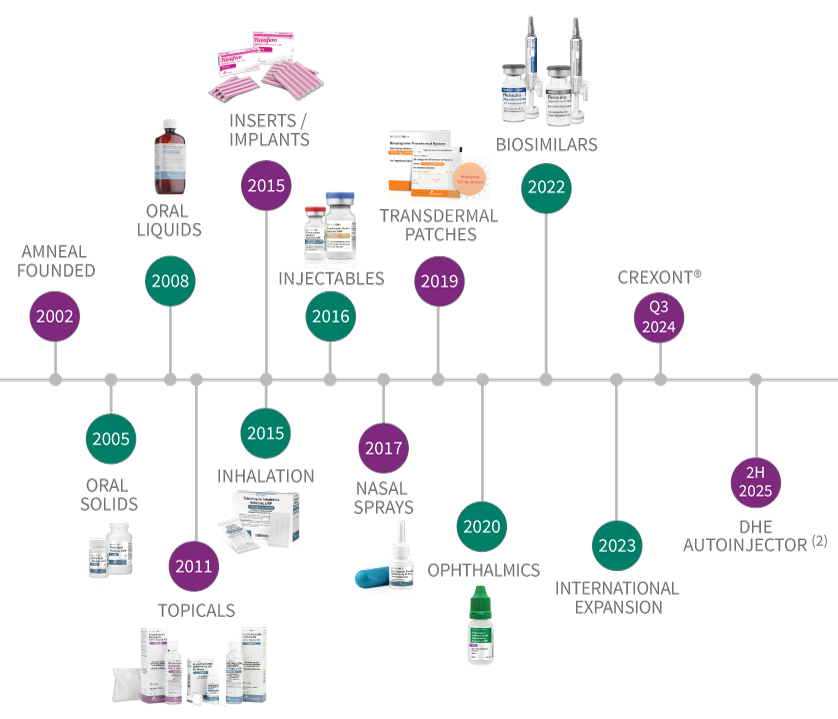
R&D Timeline
2002: Amneal Founded
2005: Oral Solids
2008: Oral Liquids
2011: Topicals
2015: Inserts/Implants, Inhalation
2016: Injectables
2017: Nasal Sprays
2019: Transdermal Patches
2020: Ophthalmics
2022: Biosimilars, LYVISPAH®
2023: International Expansion
Q3 2024: CREXONT®
2H 2025: DHE Autoinjector (2)
Note: Data as of February 28, 2025
(1) Pipeline includes investigational products not approved by FDA. Any such expected launch is subject to certain assumptions and factors, many of which are outside our control, such as regulatory approval, and may be subject to change.
(2) Pending U.S FDA Approval.
Learn more about our Specialty R&D pipeline here


