GRANDE®


GRANDE® technology is an advanced gastric retention system (GRS) that enables controlled, once-daily dosing by retaining the drug in the stomach and slowly releasing the drug for optimum absorption.
Many oral drugs have pH-dependent solubility and/or site-specific absorption in the upper gastrointestinal (GI) tract. These drugs require frequent dosing throughout the day, which can result in side effects associated with drug peak levels and poor disease control with drug trough levels.
Amneal has developed two advanced gastric retention oral drug delivery systems for insoluble and soluble drugs to retain the drug in the stomach for an extended period of time for maximum absorption during the day. The goal of the gastric retention system is to provide consistent drug release and absorption for once or twice-daily dosing for improved efficacy, tolerability, compliance, and patient satisfaction. Amneal’s gastric retention system is designed for rapid floating and swelling of the tablet in the stomach so that the dosage form stays in the stomach for an extended time, providing controlled, sustained drug delivery.
The key features of products using our GRS technologies are:
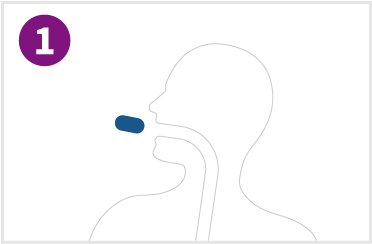
Initial tablet size is small enough for swallowing and passage into the stomach
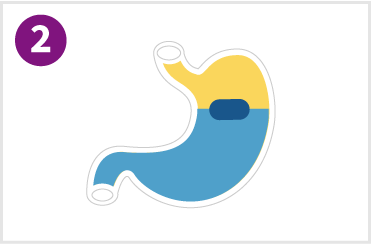
Tablet absorbs gastric fluids in the stomach and starts floating and swelling to double its size in approximately 15-30 minutes
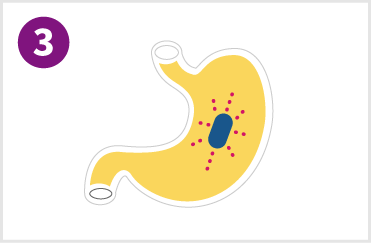
Tablet is retained in the stomach for 12-18 hours and continuously releases the drug
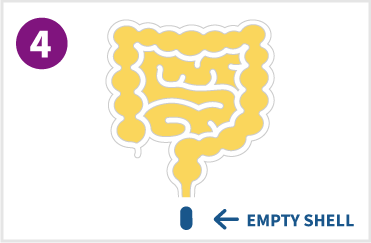
After drug release is complete, the empty tablet shell collapses or breaks into pieces and is eliminated from the body
